OLCARE LABORATORIES is committed to produce pharmaceutical formulations under stringent quality control norms , following Quality Assurance systems and in compliance with Good Manufacturing Practices during all the processes of manufacturing as we understand our ultimate aim is to “SAVE THE LIFE OF HUMAN BEINGS”. Maintaining the HYGIENE and SAFE environment is first duty of all the employees of Olcare Laboratories. It is the policy of Olcare Laboratories to meet all commitments to the best of our ability , which shall be improved continuously through training and education.
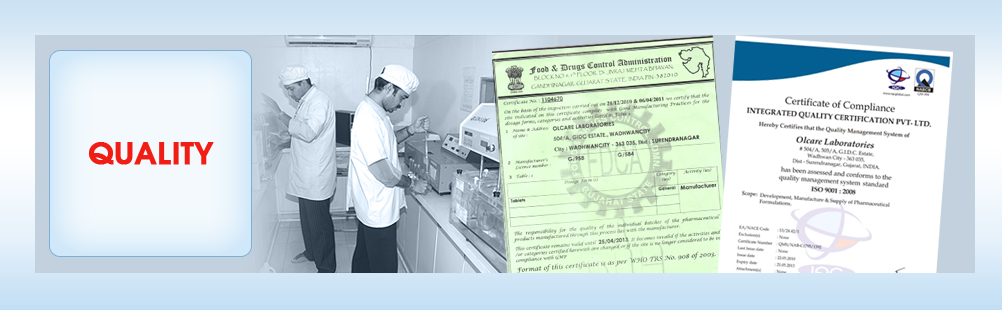
Our Accolades and Certifications!
The WHO-cGMP is used by pharmaceutical regulators and the pharmaceutical industry in over one hundred countriesworldwide. “CurrentGood manufacturing practice” or “cGMP” is part of a quality system covering the manufacture and testing of pharmaceutical dosage forms or drugs and active pharmaceutical ingredients, and pharmaceutical products. GMPs are guidance that outline the aspects of production and testing that can impact the quality of a product.
ISO-9001-2008 is a certification by International Organization for Standardization. This is awarded for maintain International standards in a Business. These standards are checked very strictly and routinely by team of independent experts and ten approved. This speaks about the systems we follow to run the organization most effectively keeping customers in focus.
NSIC D&B SMERA Rating Awarded to Olcare Laboratories is a comprehensive assessment of the enterprise taking into considerations the overall Financial and Non-Financial performance of the company. SMERA’s objective is to provide Enterprise ratings that are comprehensive, transparent and reliable to facilitate easier and adequate flow of credit from the banking sector to MSME’s.



